Dendritic cell vaccines for breast cancer
Our laboratory has previously shown loss of HER-2 Th1 immune responses in HER-2 positive breast cancer patients versus healthy adult women. This loss of immune response is associated with less responsiveness to currently available therapies and correlates with poor clinical prognosis in patients. Administration of HER2 dendritic cell vaccine has been shown to restore this immune response in HER2 positive breast cancer patients. Our lab monitors the immune responses of patients throughout treatment to help better understand the importance of HER-2 Th1 immune responses during treatment and to help further develop cellular therapies for breast cancer. We are currently developing a vaccine to generate CD4 Th1 immune response to other oncodrivers such as c-MET, EGFR, MUC1 and HER3. These oncodrivers are critical to breast cancer cell proliferation and survival in different breast cancer subtypes.
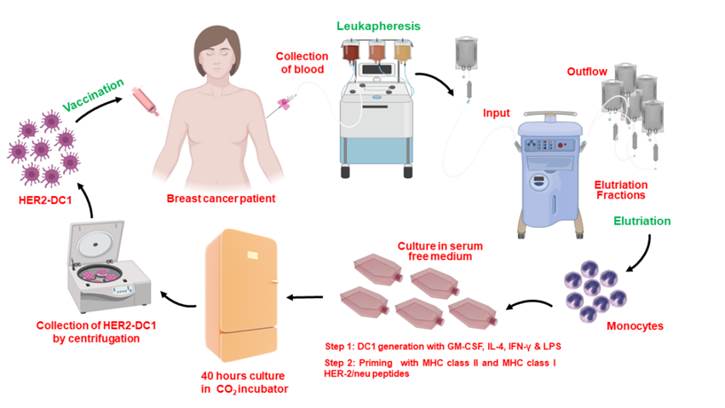
Discovery MHC Class II peptides derived from oncodrivers and CD4 activation signalling in breast cancer
The identification of immunogenic epitopes from tumor antigens has become crucial in the creation of epitope-based therapeutic and preventive vaccines. Computer-based algorithms have allowed for the identification of sequence motifs capable of binding to MHC-I and II molecules to predict the existence of CD8 and CD4 T cell epitopes within the sequences of known tumor antigens. While MHC class I prediction algorithms have shown success in identifying immunogenic class I epitopes, limited success has been seen in the identification of MHC class II epitopes. The peptide screening methodology developed in our laboratory gives a novel experimental approach for the identification of promiscuous class II epitopes. Sensitization of mature DC1s with an overlapping tumor-antigen derived peptide library allows for the identification of immunogenic peptide sequences with the ability to stimulate antigen-specific CD4+ Th1 immune responses.
DC1 and CD4 T cell subset development and activation signals for breast cancer therapy
Treatment of patients with metastatic breast cancer with current chemotherapeutic agents has failed to improve outcomes. Our laboratory has demonstrated that intra-tumoral delivery of tumor antigen pulsed DC1 combined with targeted agents, resulted in elimination of tumors in mice. This result was dependent on the interaction of antigen pulsed DC1 with anti-tumor specific CD4 Th1 cells. Many breast cancer patients lack tumor residing CD4 Th1 cells thus affecting this critical DC1 /CD4 Th1 anti-tumor response. We are currently investigating whether delivery of both anti-tumor specific CD4 Th1 cells and tumor antigen pulsed DC1 can drive robust systemic anti-tumor immunity to completely eliminate metastatic breast tumors and the mechanism by which this happens.

Adoptive T Cell Therapy
While survival in patients with early HER2+ invasive breast cancer (IBC) has improved as a result of HER2 targeted agents, patients with metastatic HER2 breast cancer (MBC) often become resistant to therapy and eventually recur. Thus, there is a strong need for novel approaches to treat metastatic HER2+ BC. We have demonstrated that in patients with recurrence there is a loss of anti-HER2 CD4 Th1 response and have shown that Th1 cytokines cause induction of tumor senescence and apoptosis in HER2 IBC, especially when combined with anti-HER2 antibodies. We have assessed whether anti-HER2 CD4 Th1 therapy induced by dendritic cells could be improved using preclinical metastatic HER2 mouse models. We have developed a protocol to expand CD4 T cells from PBMC post DC vaccination and are currently investigating the adoptive T cell therapy approach using CD4+ T cells in preclinical murine models. In addition, we are validating different methodologies for the expansion of T cells ex vivo and for improving the homing, persistence and cytotoxicity of reinfused T cells. The goal of these strategies is to improve the clinical success of adoptive T cell therapy.
Isolation and expansion of Tumor Infiltrating Lymphocytes (TIL) from breast tumors
The central theme of personalized medicine is to tailor diagnosis and treatment to a patient’s individual biologic profile. We are currently accessing the feasibility of expanding TILs from surgical breast cancer tissue and selecting ones that are reactive to the patient’s tumor. These will then be expanded and used in adoptive T cell therapy. Specifically, whole exome sequencing will be used to identify tumor-specific neoantigens from surgical specimens. Subsequently, dendritic cells (DC1) loaded with these tumor-specific neoantigens will be co-cultured with the isolated TILs. Tumor reactive TILs will be isolated, expanded and be infused back into the patient.

Requirements for preventive vaccines for breast cancer
Breast cancer prevention remains the ultimate cost-effective method to reduce the global burden of invasive breast cancer (IBC). To date, surgery and chemoprevention remain the main risk-reducing modalities for those with hereditary or high risk cancer syndromes. Ductal carcinoma in situ (DCIS) is a preinvasive malignant lesion of the breast that closely mirrors IBC and, if left untreated, develops into IBC. Even after surgical resection, patients with high-risk DCIS have a 25% increased risk for developing recurrent DCIS or IBC. Vaccination strategies to prevent or interrupt breast cancer development from the preinvasive or premalignant stage can be an ideal approach. Over expression of the oncodriver HER2, a protein receptor expressed on breast cells, is a poor prognostic indicator. We have demonstrated that a dendritic cell vaccine targeting HER2 expressing DCIS was able to elicit a strong anti-tumor Th1 immune response. Immunotherapy is well tolerated and has numerous advantages over drug-based chemoprevention. Currently, we are developing dendritic cell vaccines to target other oncodrivers, growth factors and neoantigens that are selectively expressed in preinvasive or premalignant breast lesions, with the goal of preventing development of IBC.
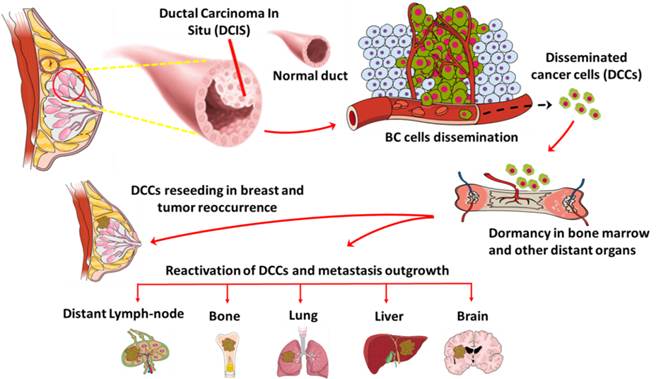
Anti-oncodriver Th1 immunity to target disseminated cancer cells
Metastatic spread in breast cancer patients is the major driver of cancer related death. A unique subset of cells disseminated from early stage breast cancer patients act as an intermediary in the metastasis outgrowth process. Approximately 40% of early stage breast cancer patients are positive for disseminated cancer cells (DCCs) in distant organs. After dissemination, DCCs enters a dormancy stage and become refractory to conventional therapies. After a prolonged period of time, these DCCs get activated and can form overt metastasis. DCCs exhibits heterogenic characteristics, stem cell like properties and express unique gene signatures, which makes them difficult to target with current therapies. Our laboratory focuses on understanding the molecular mechanisms of breast cancer cell dissemination and gene expression signatures during breast cancer progression. In addition, we are currently investigating the role of anti-oncodriver Th1 immunity and targeted therapy on DCCs and their effect on stemness/dormancy potential and tumor recurrence/metastasis seeding potential.
Immunotherapy for TNBC
Triple negative breast cancer (TNBC) is the most aggressive subtype of breast cancer and demands novel therapeutic strategy for better efficacy and improved patient outcome. One of the primary interests in our lab is to understand the molecular architecture of triple negative tumors and to develop an effective and targeted immunotherapy for TNBC patients. Breast cancer cells have multiple proteins known as oncodrivers, which play a critical role in tumor cell growth and proliferation. Developing new cancer therapies targeting these oncodriver proteins and their signaling pathways has been a promising area of translational cancer research.
Mechanisms of TH1 cytokine activation in cancer
HER2-targeted agents improve survival in early breast cancer (BC), but for patients with locally advanced or metastatic disease, therapy resistance remains a significant clinical problem. HER2 overexpression is partially maintained because E3 ubiquitin ligase Cullin5 (CUL5), which degrades HER2, is frequently mutated or underexpressed, while the client-protective co-chaperones cell division cycle 37 (Cdc37) and heat shock protein 90 (Hsp90) are increased translating to diminished survival. The Th1 cytokine interferon (IFN)-γ caused increased CUL5 expression and marked dissociation of both Cdc37 and Hsp90 from HER2, causing significant surface loss of HER2, diminished growth, and induction of tumor senescence. IFN-γ synergized with multiple HER2-targeted agents to decrease surface HER2 expression, resulting in decreased tumor growth. These data suggest a novel function of IFN-γ that regulates HER2 through the PDP pathway and provides an opportunity to impact HER2 responses through anti-tumor immunity. We are currently investigating the relationship between PDP/ HER2 targeted therapy, and the Th1 immune response in HER2 breast cancer.

Solving the brain metastasis problem using immunotherapy
In advanced breast cancer, metastatic tumors can be found in the brain and predicts a poor prognosis. At this time, there are very few treatment options for these patients. The efficacy of systemically delivered conventional therapies is limited by the relative impermeability of the blood-brain barrier or the blood cerebral spinal fluid barrier. We have demonstrated that the delivery of tumor antigen pulsed DC1 into the cerebral spinal fluid of mice bearing leptomeningeal disease was safe and effective in completely eliminat the tumors, resulting in increased survival. Currently, we are investigating whether tumor antigen pulsed DC1 in combination with other targeted agents will increase the treatment efficacy in brain metastasis models for breast cancer, melanoma and lung cancers.
Microbiome and breast cancer
Apart from inherited susceptibility such as BRCA mutations, several environmental factors and lifestyle components have also been strongly linked to breast cancer. Epidemiologic studies suggest that the human microflora contributes to 16–18% or more of worldwide malignancies. Microbiota of women with breast cancer differs from that of healthy women, indicating that certain bacteria may be associated with cancer development and in response to therapy. Our laboratory is currently investigating how the gut microbiome contributes to both breast cancer growth and survival through reciprocal interaction with the immune response.
