Generation of BMDCs
- Complete RPMI: RPMI 1640 w/ L-Glutamine and 25 mM HEPES; +10% FCS; +nonessential amino acids (from 100x stock); + Sodium Pyruvate (from 100x stock) +penicillin/streptomycin (from 100x stock); + 55 uM B-ME (from 1000x stock).
- Recombinant human Flt3L-Fc (BioXcell; 0.5 mg/ml stock; use at 1/5000 dilution)
- Recombinant murine GM-CSF (Peprotech; 10 ug/ml stock kept in aliquots at -20C. Use at 1/3000.
- CO2 euthanasia, remove femurs/tibias and clean off muscle. Cut off the top end of each bone.
- Puncture hole in 0.5 ml thin wall tube with 20 to 22 gauge needle (gauge size is important). Place bone facing down in the 0.5 ml tube, and then place tube into a 1.5 ml tube containing 100 ul of RPMI. Spin down for 1 min at 4000 G in a microfuge.
- Recover cells, centrifuge, and resuspend in 10 mls of BD PharmLyse (1/10 from 10X stock using ddH2O). Spin down immediately for 5 min.
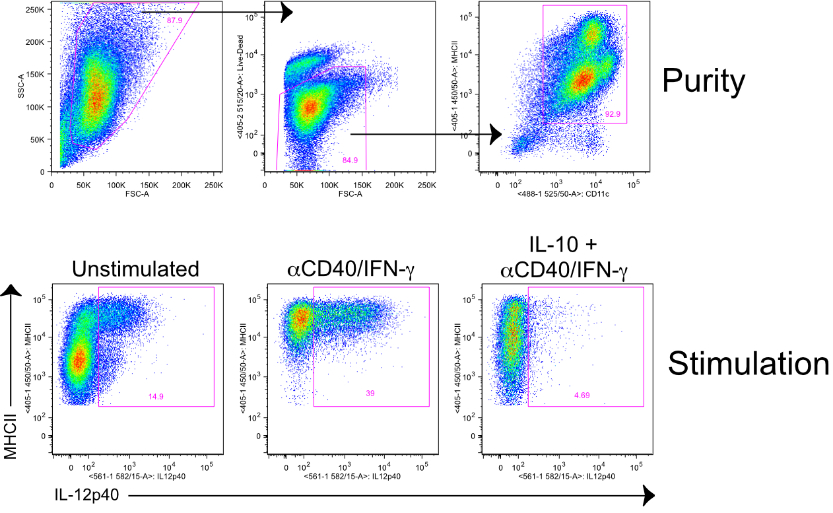
- Remove supernatant and resuspend in 20 mls of DC media. See instructions below for generating either CD11b+ or CD103+ BMDCs.
Generation of CD11b+ BMDCs
- Count and dilute to 2x106 cells/ml in media containing 100 ng/ml Flt3L-Fc. Expect 3-4 x107 cells per mouse.
- Plate cells at 5 ml/well in 6 well plates.
- Culture for 7-8 days without disturbing. Expect 0.8-1.06 per ml with >90% purity.
Generation of CD103+ BMDCs
- Count and dilute to 1.5x107 cells in 10 ml of media containing 200 ng/ml Flt3L-Fc and 3 ng/ml of GM-CSF.
- On day 5 or 6, add 5 ml of complete media (with FLT3L-Fc and GM-CSF).
- On day 9, harvest the non-adherent cells. Count and re-plate at 3x106 in 10 ml of complete media (with FLT3L-Fc and GM-CSF).
- On day 15, harvest non-adherent cells. Expect 90%+ of the cells to be positive for CD11c and CD103.