The link between metabolism and cancer has long been apparent in epidemiological studies associating obesity and lack of exercise with the disease, and even widely used as a tool for cancer diagnostics. However, only in the last decade the complex molecular mechanisms governing this intricate relationship have started to be appreciated.
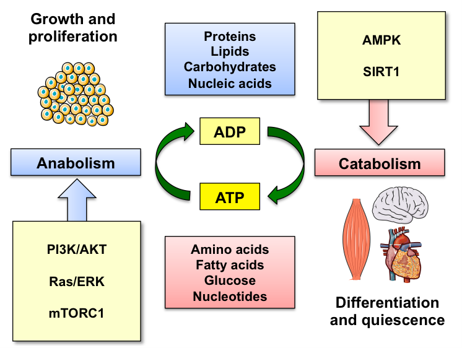
In multicellular organisms, individual cells have evolved to sense external and internal cues in order to maintain cellular homeostasis and survive under different environmental conditions. Cells efficiently adjust their metabolism to reflect the abundance of nutrients, energy and growth factors. The ability to rewire cellular metabolism between anabolic and catabolic processes is crucial for cells to thrive. This paradigm is central to the tumorigenic process as a cancer cell is under an enormous amount of metabolic strain having to cope not only with the increased energetic demand to fuel cancer growth, but also to survive the waste products of increased metabolic demand and the pressures of a hostile extracellular environment. While we still don’t understand the extent of this reprogramming and how these pathways are regulated by oncogenic signaling in tumor cells and other cellular components of the tumor microenvironment, one thing is for sure: metabolic reactions fuel the tumorigenic process. The work in the Gomes lab harnesses the knowledge acquired through the aging studies as a discovery platform for novel metabolic and redox requirements of the tumorigenic process with the goal of uncovering new actionable pathways to develop more effective anti-cancer therapeutic strategies.
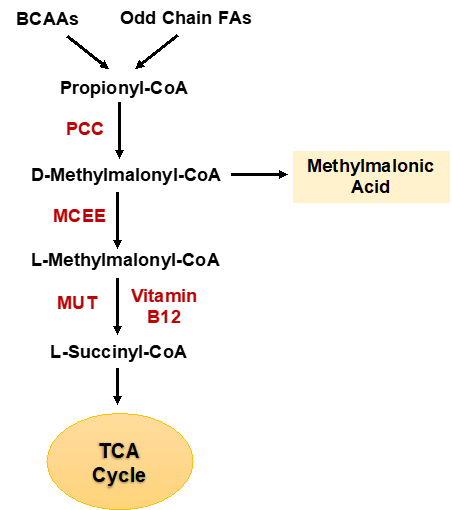
Example of this approach is our discovery that methylmalonic acid (MMA), in addition to being increased in the serum of the old, is also a metabolic adaptation of triple negative breast cancer (TNBC) essential for its metastatic colonization. We have discovered that MMA’s production is upregulated by classical metastatic signaling during TNBC’s progression and its levels dictate the ability of TNBCs to form metastatic colonies.
Questions of Interest:
How does methylmalonic acid regulate tumor progression?
Do pathways that produce and/or consume NAD(H) and NADP(H) influence tumor progression?
How does aging affect the metabolic crosstalk between the different cellular components of the tumor microenvironment?