BCR Pathway inhibitor regulation of the CLL Microenvironment
We are studying the effects of clinically available BCR Pathway specific kinase inhibitors, PI3Ki and BTKi, and how they augment the regulatory microenvironments, specifically T-regs and MDSCs, involved in CLL preservaton.
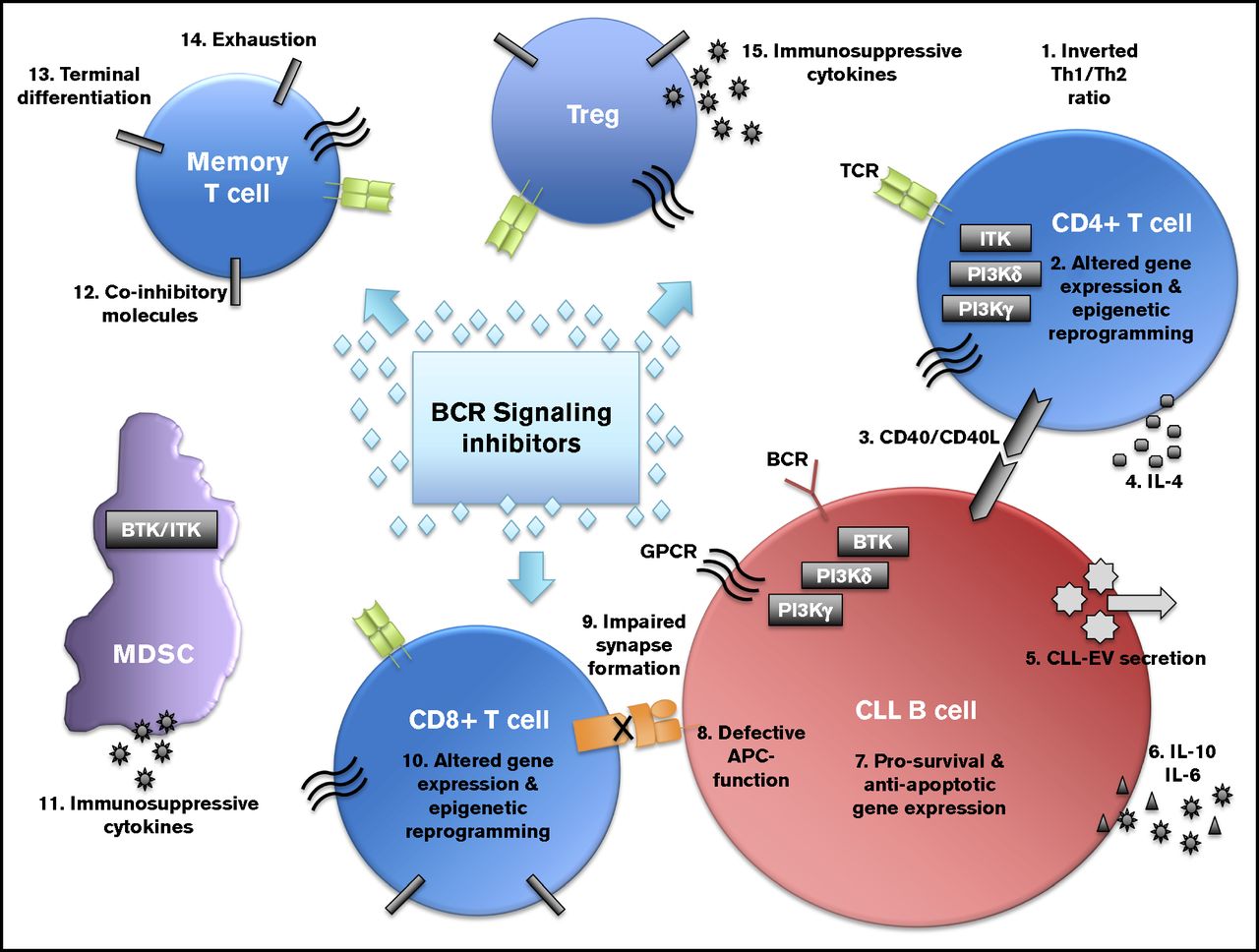
Mitochondrial Dysfunction in CLL T cells
Using the CLL Eμ-TCL1 murine model, we identified accumulation of defective depolarized mitochondria in T cells correlated with their exhausted-like phenotype.
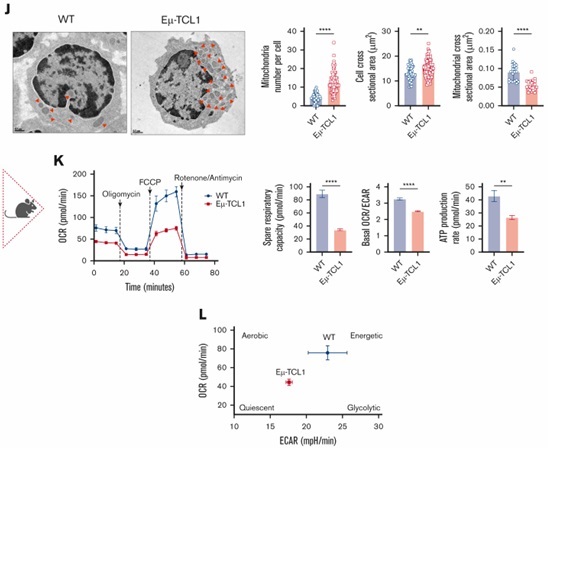
Enhancing CAR T-cell Persistence for CLL
Ex vivo reprogramming of CLL T-cell differentiation and mitochondrial activity using PI3Kδ inhibition enhances CAR T-cell efficacy and persistence in an immunocompetent murine model
![PI3Kδ inhibition allows for persistent CAR T cells in CLL in vivo. (A) Experimental design for the generation and ex vivo reprogramming of AT Eμ-TCL1 CAR T cells using idelalisib (idela), followed by infusion into different groups of AT Eμ-TCL1 mice with established disease. (B) Percentages of GFP+ CAR T cells (of total T cells) in peripheral blood (PB) for 7 weeks since the start of CLL AT in mice (arrow pointing to the time point when cyclophosphamide [CTX] and CAR T cells were injected). (C) Representative dot plots showing gating on CD19+ B cells and CD3+ T cells, alongside quantification of absolute CLL cell (CD19+CD5+B220lo) counts in PB. (D) Kaplan-Meier survival analysis of CAR T-cell–treated mice compared with control mice receiving untransduced (UT) T cells. (E) Spleen analysis 4 weeks after CAR T-cell injection, showing representative spleen sizes and tabulated weights, absolute CLL cell counts, and GFP+ CAR T-cell counts. (F) Quantitative results of endogenous T-cell memory phenotypes in the spleens of treated mice (naïve, CD44–CD62L+; CM, CD44+CD62L+; EM, CD44+CD62L–; and effector, CD44–CD62L). (G) Bone marrow (BM) analysis 4 weeks after CAR T-cell injection, showing absolute CLL cell counts and GFP+ CAR T-cell counts. The experiments included 12 mice for the idela CAR T-cell group, 11 mice for the DMSO CAR T-cell group, and 9 mice for the UT control group. On week 4 after CAR T-cell infusion, 3 randomly selected mice from each group were euthanized for spleen and BM analyses. Data are representative of 2 independent experiments. Each symbol represents an individual animal. Data are presented as mean ± SD. Differences analyzed using an ordinary 1-way ANOVA with Tukey multiple correction test in panels B-C,E-G. Log-rank (Mantel-Cox) test was used for survival study comparisons. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Panel A was created with BioRender.com. AT, adoptive transfer; GFP, green fluorescent protein; ns, non-significant; Untransd, untransduced.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/10/10.1182_bloodadvances.2024014822/2/m_blooda_adv-2024-014822-gr7.jpeg?Expires=1753790418&Signature=OEleomjpywrkcQPxhngzTG~QCO1YYqh0VLM9UVc3BOjGOqbKTIxORcYD28QgVYcX-JlpxhtY0PVmvLVp-8jmZAkNvfRGlqoP-6OuO6JdU9KJIotpbG31Oqs8IEWtAfKzua7UOudZtsXQuDx8Ce80Nmr0h8lpogdRR9N5AYXkrTuIsexl4k0CDgWqUCvZ3-e3m2b3fciEw-00NLIY4JWn-Ht-8fU4L76JnyHiNvaWuwU~y77MflReSGxuZboak0W1T6yqtjbRW9A0fFQEz5ZtdMZUNC7DWdGATVvrht-JCjPCIQ~2jUpVL159jMLHlRiVVRGL2D3AWj~L2QQNXSc5YQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Role of Mitochondrial Metabolism in CLL T-cell Dysfunction
The lab is interested in investigating novel mitochondrial pathways that can potentially contribute to T-cell dysfunction in CLL

Th17 Cells for Adoptive Cell Therapy in CLL
Our data indicate the feasibility of polarizing Eμ-TCL1-derived CD4+ T cells into Th17 cells that can be of potential use for improving the efficacy and persistence of adoptive cell therapy in CLL

