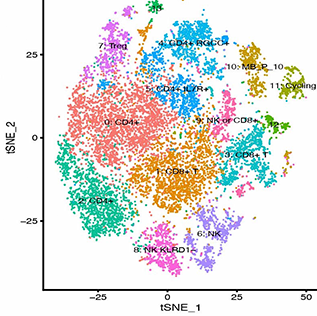
Background
Melanoma Drug Resistance and Metastasis: Although great strides have been made in treating patients having mutant BRAF-driven melanoma with BRAF and MEK inhibitors, the majority of these patients still fail therapy. Several resistance mechanisms have been identified, underscoring the heterogeneous nature of melanomas. Further, phenotypic variability exists even with genetically homogeneous populations of tumor cells, including striking variations in mRNA and protein expression at the single-cell level. This results in a wide range of cell states and phenotypes that likely explain why tumors nearly always evade therapy. The roles of phenotypic variability in progression and drug resistance are poorly understood, and we submit that strategies tackling this diversity are needed for durable therapeutic responses. We hypothesize that: drug-induced tumor cell phenotypic heterogeneity is driven by the development of inter-dependent and cooperative metabolic niches that promote tumor dissemination and affect the response to therapeutics; and therapeutic approaches that exploit intra-clonal metabolic competition will give more durable responses in melanoma patients.
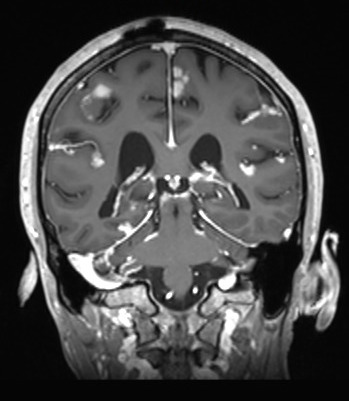
Metastasis to the Central Nervous System: As we have improved patient survival with more efficacious targeted and immune-checkpoint blokade therapies, we have observed a trend towards increased incidence of central nervous system (CNS) metastasis, including metastasis to the leptomeninges. Leptomeningeal metastasis is a poorly understood and rapidly fatal complication of many cancers (median survival 6-8 weeks) in which tumor cells metastasize to the CSF space, either hematogenously, with a putative CNS entry site via the choroid plexus, by direct extension from CNS metastases or retrogradely along peripheral nerves. The biology of this process is entirely unknown and remains unexplored due to many technical difficulties, including the lack of access to site of tumor and lack of in vitro and in vivo models for this disease. In several cancer types, (i.e. melanoma, breast, lung, and lymphoma) the systemic tumors can be well controlled therapeutically but the CSF is the sole site of relapse. We hypothesize the meninges might act as a “sanctuary” site for cells on therapy. At this time, there are no efficacious FDA approved therapies specific for the treatment of metastasis to this site. Furthermore, diagnosis of this disease is a clinical challenge, even the gold standard for diagnosis results in a false-negative 50% of the time.
Metabolic Crosstalk in the Tumor Microenvironment: Traditionally metabolism has been viewed as a collection of catabolic and anabolic pathways that generate energy and biosynthetic precursors required for growth and survival. However, emerging evidence suggest broader roles for metabolic processes in controlling other aspects of physiology, including immune cell effector functions. Another goal of our research program is to define how metabolic heterogeneity of the tumor microenvironment promotes tumor growth and survival in the CNS.
Ongoing Research Projects
Project Focus 1: The role of metabolic heterogeneity in melanoma dissemination and therapy escape.
Project Focus 2: Dissecting the tumor microenvironment of leptomeningeal melanoma metastasis through spatial multi-omic analysis.
Project Focus 3: Improving the understanding and detection of leptomeningeal metastasis in breast cancer, melanoma, and lymphoma.
Project Focus 4: Roles of the metabolic landscape of TME in melanoma tumor growth and survival in the CNS
Novel Technologies
- Single-cell RNAseq and single-cell metabolomics (separate and in the same cell)
- Integrated spatial RNAseq and spatial lipidomics
- Biochemical diagnostic tools for enhanced detection sensitivity
- In vitro and n vivo models of brain metastasis and leptomeningeal metastasis
Join our team! We are currently recruiting graduate students, postdocs and research assistants. For more information visit the Opportunities page.