Control of cellular serine metabolism by NRF2 supports proliferation and antioxidant defense
Suppression of ROS is not the only mechanism by which NRF2 influences tumorigenesis. We found that NRF2 promotes the activity of the serine biosynthesis pathway, which is required for the in vitro and in vivo growth of lung cancer cells with high NRF2 activity (DeNicola et al., Nat Genet 2015). Importantly, we found that serine synthesis is dispensable for the health of mice under nonstressed conditions (Kang et al. Cancer Metab 2020), suggesting that targeting serine synthesis will be tumor-selective and may have therapeutic benefits in patients.
NRF2 rewiring of cancer metabolism confers metabolic liabilities
While the regulation of serine synthesis favors proliferation and survival, we found that not all NRF2-regulated processes are favorable. Using our genetically engineered mouse models of NRF2 activation, we found that NRF2 promotes the accumulation of intracellular cysteine and engages the cysteine homeostatic control mechanism mediated by cysteine dioxygenase 1 (CDO1). An enzyme in the taurine synthesis pathway, CDO1 limits cysteine accumulation by catalyzing the irreversible metabolism of cysteine to taurine synthesis precursors. In NRF2 active cells, this reaction produces toxic and wasteful metabolites, consequently leading to the epigenetic silencing of CDO1 in human non-small cell lung cancer to alleviate this detrimental metabolism. Thus, CDO1 is a metabolic liability for NSCLC cells with high intracellular cysteine, particularly NRF2/KEAP1 mutant cells (Kang et al., Elife 2019).
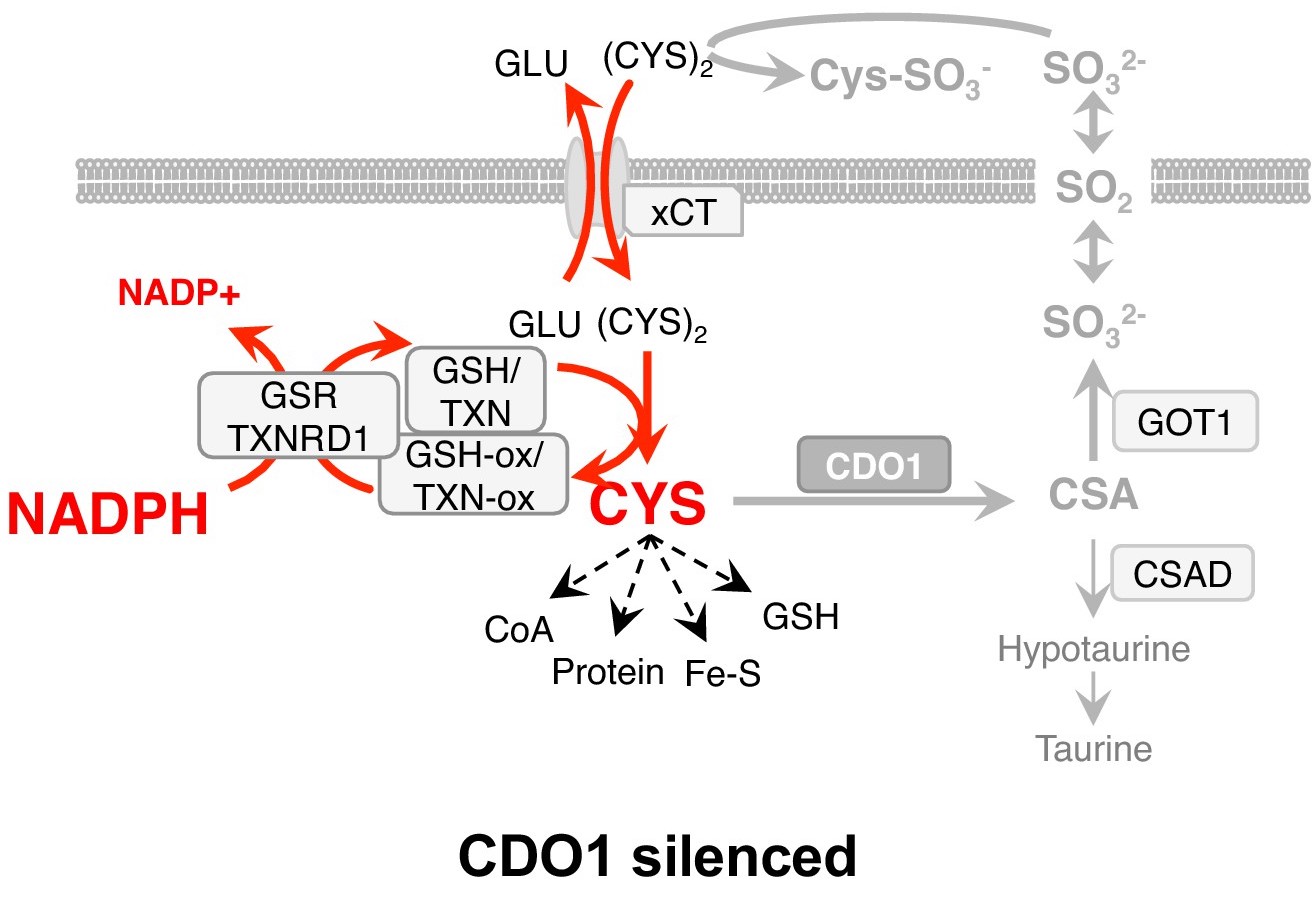
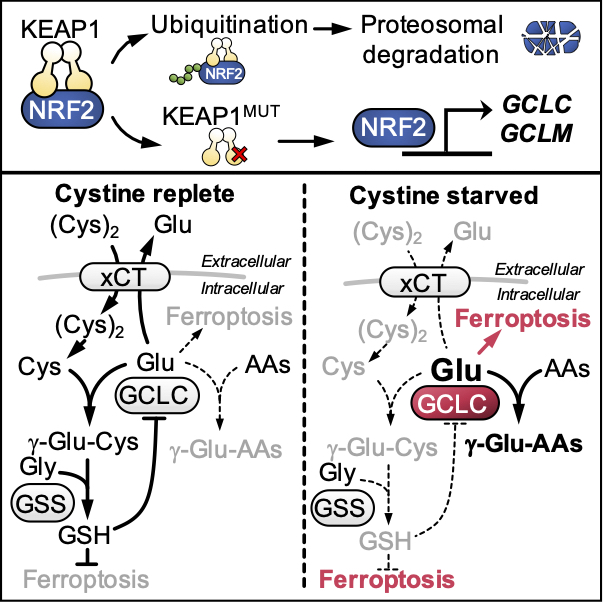
Cystine starvation promotes a non-canonical function of the glutathione synthesis machinery
NRF2-mediated metabolism also promotes cell survival. NRF2 controls the synthesis of glutathione to protect against ROS. Surprisingly, we also identified an essential, non-canonical function of the glutathione synthesis enzyme GCLC under cystine starvation conditions. Under replete conditions, GCLC ligates glutamate to cysteine in the first step of glutathione synthesis. When cysteine is limiting, we found that GCLC instead ligates glutamate to alternative amino acids to prevent the toxic accumulation of glutamate and ROS generation. KEAP1/NRF2 mutant cells are protected against cysteine-starvation induced ferroptosis due to elevated GCLC expression and limited glutamate accumulation, and dual targeting of cystine import and GCLC activity can overcome the resistance of NRF2 active cells to ferroptosis (Kang et al., Cell Metab 2021). Importantly, these findings demonstrate that while the depletion of glutamate by GCLC is a metabolic vulnerability under nutrient replete conditions, we find that it confers an advantage under cysteine starved conditions.
Open questions: